Operando X-Ray Absorption Spectroscopy reveals different Hydrogen dissociation pathways on model Cu:CeO2 and Fe:CeO2 surfaces
The search for new materials that can substitute expensive Pt-based catalysts in electrodes of electrochemical devices has been an increasingly relevant topic in the past years, with the aim to introduce cheaper elements and move toward a more sustainable industrial production and economy.
Among materials suitable as alternative catalysts, reducible oxides such as CeO2 are particularly interesting because of their ability to store and release oxygen depending on the surrounding environment.
A valuable route to improve the catalytic behavior of CeO2 is the direct oxide modification through the inclusion of low-valent metal cations, which can act both as a center for hydrogen dissociation and for the introduction in the oxide of charge redistributions that increase the ability to exchange oxygen and electrons with the environment, lower the reaction energy barriers, and favor oxygen vacancy formation. In particular, Cu inclusion increases the oxide reducibility already at 470 K, lowering the CeO2 reaction temperature.
This study exploited the combination of operando near-edge X-ray absorption fine structure (NEXAFS) technique and gas chromatography to correlate the changes of the chemical state of Cu and Ce cations on 5 nm films with oxygen vacancy formation and water desorption in real time in the temperature range of 300–620 K in an ambient pressure of hydrogen.
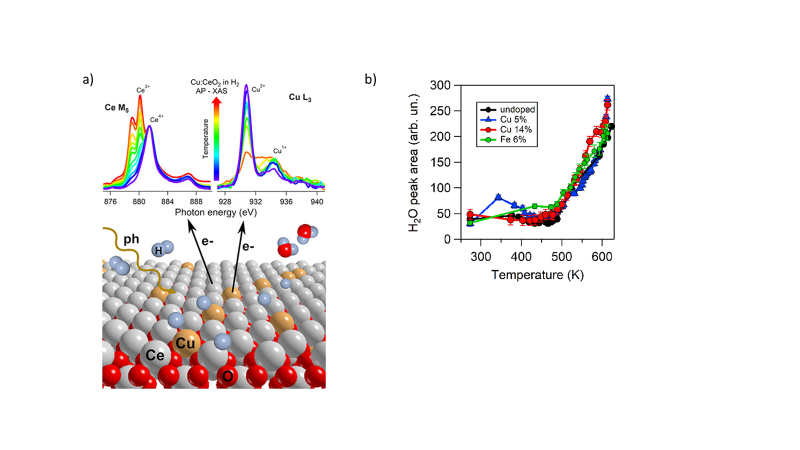
Pure and Cu- and Fe-doped CeO2 films were investigated during heating in H2 flow to unravel the modifications in the cation oxidation states and the process occurring in a reducing environment in real conditions, exploiting the operando NEXAFS reaction cell developed at APE-HE beamline of Elettra Synchrotron. The results show that CeO2 reduction process is favored by the inclusion in the matrix of Cu ions, while Fe doping seems not to increase the CeO2 activity. The simultaneous monitoring of Cu and Ce oxidation states during the reaction and of water desorption (Figure 1) allowed to depict the process occurring on the surface of the doped ceria, suggesting an increased reducibility supported by the presence of Cu1+ that remains active also when modifications are not anymore observed in the Cu oxidation state.
The investigation allowed to obtain important insights about the reaction mechanism: below 500 K, Cu1+ sites located on the surface or at grain boundaries favor H2 dissociation, while the electrons donated by H contribute to reducing further Cu2+ atoms and Ce4+ to Ce3+ (see figure 1). H atoms remain on the surface forming OH groups, and no water is desorbed. Above 500 K, no further Cu reduction occurs, Ce continues reducing, and water is desorbed from the surface, indicating a stronger surface activity compared to pure CeO2. On the contrary, the inclusion of the Fe dopant does not modify the Ce reducibility, while it increases the extent of O vacancy formation limited to low temperatures compared to pure ceria because of a nondirect role in H2 adsorption and dissociation.